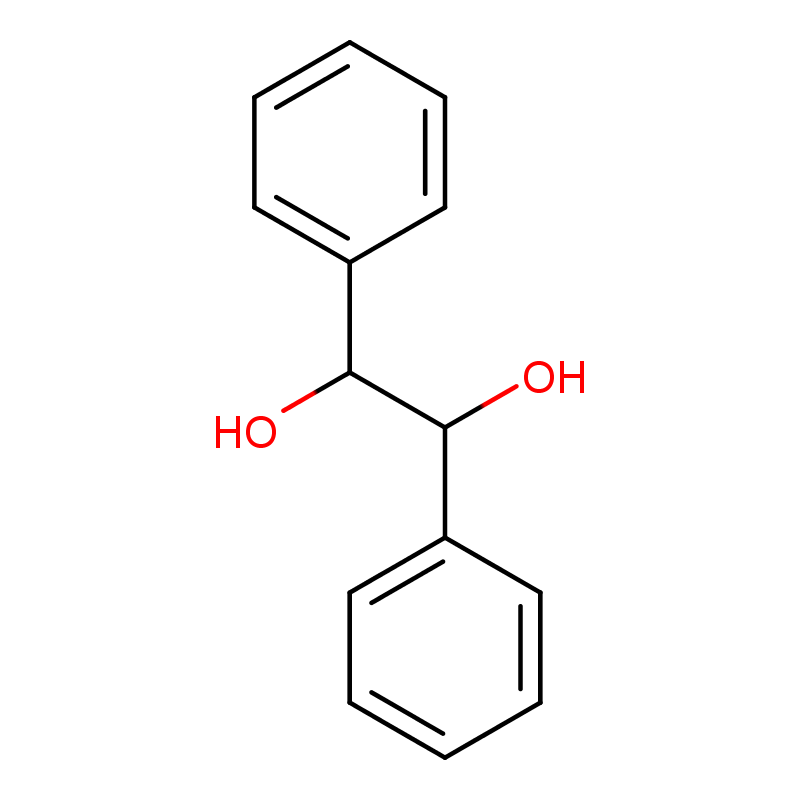
(S,S)-(-)-Hydrobenzoin Manufacturer CAS No.: 2325-10-2
Cas No.:2325-10-2
Purity: 97%min
Molecular formula: C14H14O2
Appearance: light yellow to white solid</p>
Short description:
Certificate of Analysis:
| Item | Index | Test Result |
|---|---|---|
| Appearance | light yellow to white solid | light yellow to white solid |
| Identification (H-NMR) | positive | conforms |
| Purity (HPLC) | >97% | 98.00% |
| Chiral Purity(e.e.%) | >97% | 99.80% |
| Conclusion | Approved |
What is (S,S)-(-)-Hydrobenzoin Cas No.: 2325-10-2 ?
(S,S)-(-)-Hydrobenzoin CAS No.: 2325-10-2 is a chiral organic compound that serves as a crucial intermediate in various synthetic processes, especially in asymmetric synthesis and as a resolving agent in stereochemistry.
Structure and Properties
- Chemical Formula: C14H14O2
- IUPAC Name: (1S,2S)-1,2-Diphenylethane-1,2-diol
- Stereochemistry: The “(S,S)” notation indicates that the molecule has two stereogenic centers, both with the same (S)-configuration, which gives the compound its specific three-dimensional arrangement.
- Appearance: Typically, (S,S)-(-)-Hydrobenzoin appears as a white crystalline solid.
- Chirality: The molecule is chiral due to the presence of two hydroxyl (-OH) groups attached to the carbon atoms, each bonded to phenyl groups, creating a pair of asymmetric centers.
Main Applications
- Chiral Auxiliary and Ligand:
- Asymmetric Synthesis: (S,S)-(-)-Hydrobenzoin is widely used in asymmetric synthesis. It can serve as a chiral auxiliary, where its stereochemistry helps induce chirality in the synthesis of other compounds.
- Catalysis: It can act as a chiral ligand in various catalytic processes, aiding in the formation of enantiomerically pure products.
- Resolution of Racemic Mixtures:
- Resolving Agent: We use this compound to resolve racemic mixtures of other substances into their enantiomers. It forms diastereomeric complexes with racemic mixtures, allowing for the separation of enantiomers based on their different physical properties.
- Pharmaceuticals:
- Drug Synthesis: (S,S)-(-)-Hydrobenzoin is a precursor or intermediate in the synthesis of various chiral drugs. Its stereochemistry is crucial in producing the desired enantiomerically pure pharmaceuticals.
- Stereochemical Integrity: The specific (S,S) configuration is often important for the biological activity of the target drugs, ensuring they interact correctly with chiral biological targets.
- Analytical Chemistry:
- Reference Compound: In chiral chromatography, (S,S)-(-)-Hydrobenzoin can be used as a reference standard to determine the enantiomeric purity of other compounds.
- Method Development: It aids in the development and validation of methods for chiral separation and analysis.
- Synthesis of Complex Molecules:
- Organic Synthesis: In organic synthesis, it serves as a building block for creating more complex chiral molecules. Its two hydroxyl groups can be modified to form various functional groups, facilitating the synthesis of diverse compounds.
- Academic Research:
- Study of Stereochemistry: We frequently use it in research to study stereochemical effects and reactions due to its well-defined chirality and ease of handling.
Summary
(S,S)-(-)-Hydrobenzoin is a versatile chiral diol that plays a crucial role in asymmetric synthesis, the resolution of racemic mixtures, and the synthesis of enantiomerically pure pharmaceuticals. Its applications span across synthetic chemistry, catalysis, and analytical techniques, making it a valuable compound in both industrial and academic research settings.