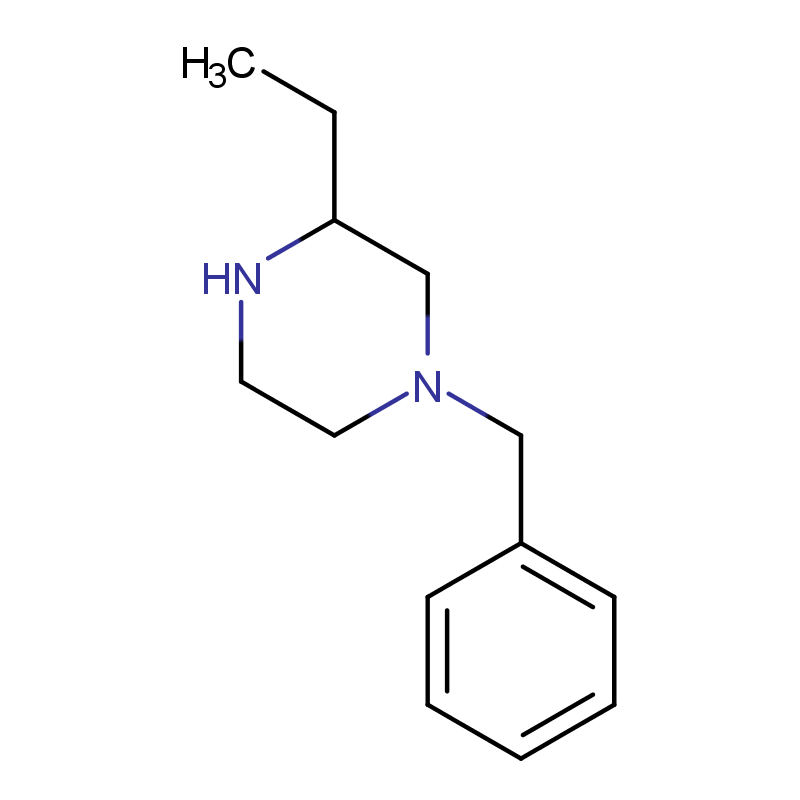
(R)-3-N-BENZYL-2-ETHYLPIPERAZINE Manufacturer CAS No.: 347195-55-5
Cas No.:347195-55-5
Purity: 98%min
Molecular formula: C13H20N2
Appearance: light yellow solid
Short description:
(R)-3-N-Benzyl-2-ethylpiperazine is a chiral organic compound belonging to the piperazine class, which is commonly utilized in various pharmaceutical and chemical research applications. Its unique structure and properties make it an important intermediate in the synthesis of complex molecules, particularly in the development of drugs and fine chemicals.
What is (R)-3-N-Benzyl-2-ethylpiperazine Cas No.: 347195-55-5 ?
(R)-3-N-Benzyl-2-ethylpiperazine is a chiral piperazine derivative with a benzyl group attached to the nitrogen atom and an ethyl group on the 2-position of the piperazine ring. The “(R)” denotes the configuration of the chiral center at the 2-position.
Main Applications:
- Pharmaceutical Intermediate:
- Drug Development: Researchers primarily use (R)-3-N-Benzyl-2-ethylpiperazine as an intermediate in the synthesis of pharmaceutical compounds. We can employ piperazines which are a versatile class of compounds in medicinal chemistry for developing drugs targeting various biological systems, including central nervous system agents, antipsychotics, and antibiotics.
- Chiral Building Block: The compound’s chiral nature makes it valuable in synthesizing enantiomerically pure substances. Enantiomerically pure drugs can offer improved efficacy and reduced side effects compared to their racemic mixtures.
- Chemical Research and Development:
- Synthetic Chemistry: In research settings, this compound serves as a building block for creating novel molecules. We use it to investigate new synthetic routes and the properties of chiral piperazine derivatives. The benzyl group on the nitrogen provides a site for further functionalization, allowing chemists to explore diverse chemical transformations.
- Chirality Studies: Researchers utilize this compound to study the effects of chirality on the biological activity of molecules. Understanding how different enantiomers interact with biological systems can lead to the development of more targeted and effective therapies.
- Catalysis and Ligand Design:
- Asymmetric Synthesis: We can use it as a ligand in asymmetric synthesis, a process crucial for producing chiral molecules in high enantiomeric excess. It can help in catalyzing reactions where the chirality of the product is influenced by the chiral environment provided by the ligand.
- Coordination Chemistry: In coordination chemistry, piperazine derivatives can act as ligands to form complexes with metals. We investigated these complexes for their potential applications in catalysis, materials science, and as therapeutic agents.
Safety and Handling:
Researchers intended this compound for research and development use and did not approve it for human or veterinary applications yet. We should handle it with appropriate safety measures to avoid exposure, as it may cause irritation or other health effects upon contact.
| Item No.: | CHL0037 |
|---|